MedGenome brings precise technology for MRD testing in India in collaboration with Adaptive Biotechnologies™
Precision NGS MRD testing with clonoSEQ

The clonoSEQ® Clonality (ID) Test identifies trackable immune receptor DNA sequence(s) associated with a lymphoid malignancy. Diagnostic (pre-treatment) samples are typically used, but other samples with high disease burden (e.g., relapse) are also acceptable.

More about clonoSEQ
With state-of-the-art MRD assessment, what gets measured gets managed.®
- SensitivityDetect MRD at low levels that correlate with patient outcomes: a single cancer cell among a million healthy cells, given sufficient sample input. This offers prognostic value to clinicians as they assess how patients respond to treatment.1,2
- SpecificityPrecisely identify and quantify tracked malignant cells. Once the DNA sequences of the B- or T-cell receptors associated with these cancer cells are identified, the level of each specific sequence can be assessed in subsequent MRD samples.1
- StandardizationEnsure consistency and reproducibility of results for patients over time. clonoSEQ has undergone extensive analytical and clinical validation, fulfilling requirements for U.S. Food and Drug Administration (FDA) clearance for in vitro diagnostic use.1,2
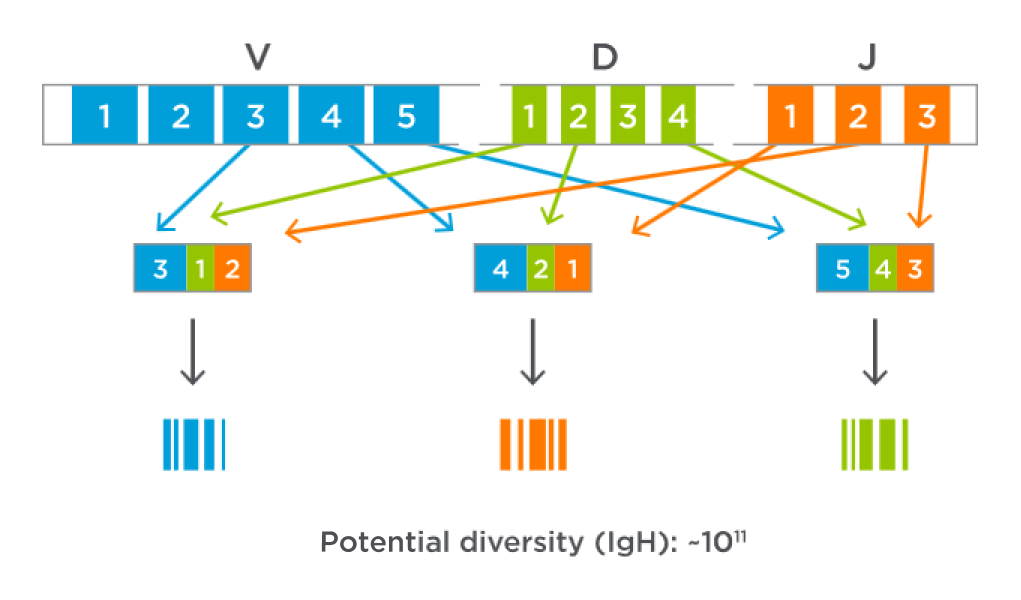
Each B- or T-cell receptor is coded by unique DNA sequences made of 3 segments—Variable, Diversity, and Joining—that serve as DNA “barcodes” that can be used to track malignant cells.1,3
Ordering clonoSEQ
To order clonoSEQ, request your order from a MedGenome representative. Please call 1800 296 9696.
clonoSEQ Clinical Reports
Clinicians receive clonoSEQ test results via an informative clinical report. clonoSEQ reports are delivered to MedGenome via secure online portal, along with an opt-in email notification to alert the Clinician / MedGenome representative when the new report is available.
- Clonality (ID) ReportIdentifies trackable DNA sequence(s) associated with malignancy in a fresh or archived high disease load sample collected at the time of diagnosis or relapse.
The Clonality (ID) Report is the first report that a clinician receives upon initiating clonoSEQ testing for a new patient. This report indicates whether one or more trackable sequences has been identified for the patient, thus enabling subsequent MRD testing. - Tracking (MRD) ReportQuantifies and tracks minimal residual disease (MRD) during or after treatment in a freshly drawn bone marrow, peripheral blood, or plasma sample.*
Each Tracking (MRD) report includes the results from previous reports, providing a visual representation of changes in disease burden over time.
clonoSEQ Reports
clonoSEQ Clinical Reports One Pager for India
References
- clonoSEQ®. [technical summary]. Seattle, WA. Adaptive Biotechnologies; 2020. https://www.clonoseq.com/technical-summary/
- Ching T, et al. BMC Cancer. 2020;20:612.
- Abbas A, et al. Cellular and Molecular Immunology. 9th ed. Elsevier; 2018.
The clonoSEQ Assay is not approved by the Drug Controller General of India (DCGI) for diagnostic use. The clonoSEQ Assay is available as an FDA-cleared in vitro diagnostic (IVD) or a CLIA-validated laboratory developed test (LDT) as a single-site assay performed at Adaptive Biotechnologies Corporation™ in Seattle, Washington, USA. This content is for the clinician’s reference use only.


 Enquire
Now
Enquire
Now