Pioneering Precision in Thyroid Tumor Testing for Informed Healthcare Decisions

ThyroTrack assay is a NGS-based sequencing assay that helps to comprehensively understand thyroid tumor genetics allowing improvement in the diagnostic accuracy and precise personalized treatments. ThyroTrack test screens variable genetic alterations in thyroid cancer-related genes curated according to ATA (2015) & NCCN (2022) guidelines, covering SNVs, Small InDels in 46 genes, and Fusions in 23 genes.

ThyroTrack
Next generation sequencing based test to detect genomic biomarkers in thyriod nodules.
46 genes*
(SNVs and InDels)
Includes BRAF, RAS and P13K Pathway genes
23 genes*
(Fusions)
Includes known and unknown fusions in RET, NTRK, ALK and other genes
Why Should You Consider ThyroTrack Test?
- Determines accurate diagnosis and informed surgery decisions in thyroid nodules with benign/indeterminate FNA cytology
- Provides informed clinical management and treatment options for malignant thyroid nodules
- Can be used pre-surgery on FNA sample and post-surgery on FFPE tissue block
Who Should Undergo ThyroTrack Testing?
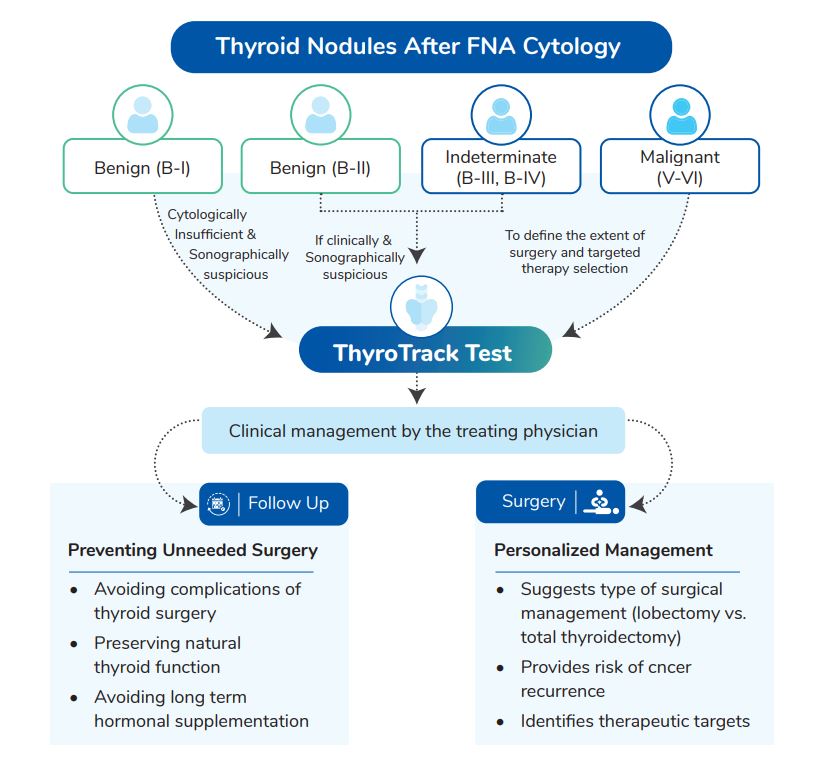
- Thyroid FNA with indeterminate cytology (Bethesda categories III and IV)
- Malignant thyroid cytology (Bethesda category V and VI), when results of the NGS are expected to affect the decision for extent of oncological surgery/treatment
- Benign thyroid cytology (Bethesda category II), when strong Suspicion of Malignancy exists on clinical grounds such as presence of a highly suspicious sonographic pattern
- Bethesda category I nodules which are cytologically insufficient and suspicious on sonographic findings
- When the diagnosis of thyroid cancer is established cytologically or histologically and molecular profiling will affect decision regarding radioactive iodine therapy, intensity of follow up, or for selection of targeted therapies in patients with advanced cancer

Gene List
| SNVs and Short InDels | |||
|---|---|---|---|
| AKT1 | CTNNB1 | EZH1 | IDH1 |
| MET | NTRK3 | PPARG | RNF213 |
| TERT | VHL | ALK | DICER1 |
| FARSB | IDH2 | NF2 | PI3K |
| PTEN | ROS1 | TG | APC |
| EIF1AX | FGFR2 | KDM6A | NRAS |
| PICALM | PTH | SLC5A5 | TP53 |
| BRAF | EP300 | GNAS | KRAS |
| NTRK1 | PIK3CA | RAF1 | STK11 |
| TSC2 | CHEK2 | ERBB4 | HRAS |
| MEN1 | NTRK2 | PIK3R2 | RET |
| SYN2 | TSHR | ||
| Fusions | |
|---|---|
| ALK | EML4 |
| FGFR2 | NTRK1 |
| PAX8 | RAF1 |
| ROS1 | THADA |
| BRAF | ERBB4 |
| KIF5B | NTRK2 |
| PICALM | RET |
| SS18 | UACA |
| CLIP1 | FARSB |
| MET | NTRK3 |
| PPARG | RNF213 |
| SYN2 | |
Assay Specification
| Sample Requirements* | FNAC Fluid in RNA later; Tissue in RNA later ; FFPE tissue block |
| FFPE Block Requirement* | Cross-sectional tumor area of 25mm2 containing at least 40 μm of tumor |
| Tumor Purity Minimum | 20% |
| Limit of Detection | 5% VAF* for SNV and INDELs >10 spanning reads for fusions |
| Panel Inclusion | 46 genes analysed for SNVs and InDels 23 genes analysed for fusions (All partners can be identified) |
| Depth of Sequencing | Average >250X |
| Analytical Sensitivity | 98% |
Test Details
| Test Code | MGM2538 |
| Test Name | ThyroTrack |
| TAT | 14 Working Days |
| Sample Type | 1) FNAC Fluid in RNA Later* Tissue in RNA Later* (Shipped at 2-4 Degree Celsius) |
| 2) FFPE Tissue Block (Shipped at Room Temperature) |
Clinical Evidences
| Positive testing for BRAF, RET/PTC or PAX8/PPARγ was specific for a malignant outcome in 100% of cases, whereas RAS mutations had an 84% risk of cancer and a 16% chance of benign follicular adenoma [PMID: 24811481]. | 462 thyroid nodules with AUS/FLUS cytology were assessed using mutation profiling; 31 were positive on mutational analysis (6.7%). 98 of the cases (21%) had a definitive diagnosis by either surgical (n=96) or non-surgical (n=2) methods [PMID: 26356635]. | In the largest prospective study of nodules with indeterminate cytology (n=653); detection of mutations was reported to convey an 88% risk of cancer among nodules with surgical follow-up; 63% of cancers on final histopathology were identified with a positive mutation preoperatively and 94% of nodules that were negative on mutation analysis had a benign final histopathology [PMID: 21880806]. |
Get Genetic Counseling with MedGenome Genetic Experts
Please share your details with our genetic experts to answer your queries
 Enquire
Now
Enquire
Now 
