What is a compendious TB Diagnostics Solution?

- A group of tests offered by MedGenome for Tuberculosis diagnosis and drug resistance profiling
- This includes:
- Conventional tests like smear and culture
- Molecular tests such as nucleic acid amplification and line probe assay
- Whole genome sequencing of Mtb for diagnosis and drug resistance profiling

Prevalence

- Worldwide, tuberculosis (TB) is the 13th leading causes of death and second leading cause from single infectious agent after COVID-19.
- According to a WHO 2020 report, Globally, an estimated 10.0 million (range, 8.9–11.0 million) people fell ill with TB with India accounting for 2.64 million TB cases (incidence + relapse) in 2019.
- As per WHO report in 2020 worldwide 5.8 million new TB cases were reported of which 1.8 million were from India.India account for almost 30% of total TB cases reported.
- India is one of the top 3 countries with the largest number of MDR/RR-TB cases that constitute 27% of global MDR/RR-TB cases.
Why do you need the test?
- A range of tests for diagnosis and drug resistance profiling
- These tests can diagnose TB as well as identify the mutations that are likely to cause drug resistance
- Multiple applications – Over and above diagnosis and drug resistance testing, the whole genome sequencing test for Mtb, due to its voluminous data availability, can be used for strain typing, epidemiology studies and disease surveillance
- The primary applications for each test or test group is as follows:
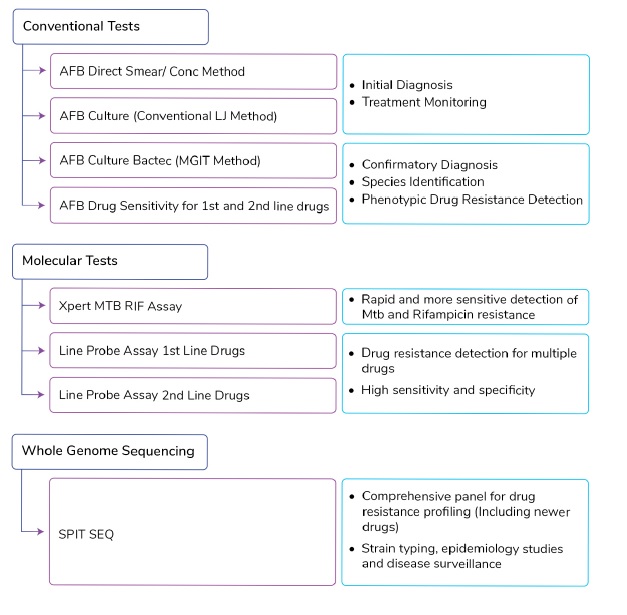
When do you need to get tested?

- The treating clinician can opt for these tests during the entire TB management cycle of the patient
- Tests like smear microscopy, culture and GeneXpert can be opted for during the initial clinical presentation
- More sophisticated tests such as Line Probe Assay can be opted for either at the beginning or during the course of TB management
Why MedGenome?
MedGenome offers a range of tests from Whole Genome Sequencing for Diagnosis and Drug Resistance Profiling of Mtb to molecular tests as well as conventional tests:
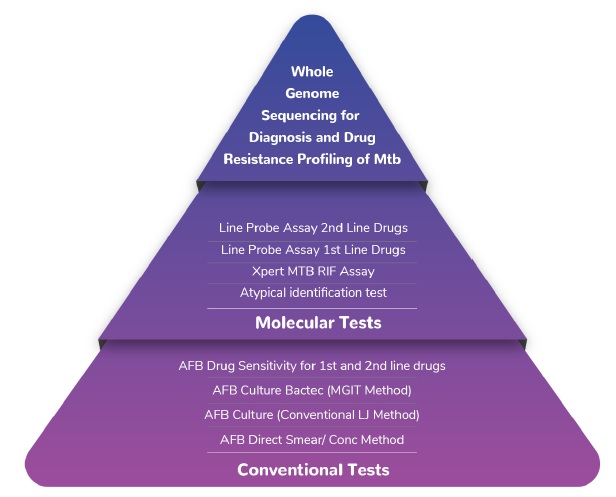
Conventional Tests:
- Proven, widely used simple diagnostic test for all types of specimens
- Fluorescent stain method as well as ZN stain method available
- This test has a sensitivity of 65% and specificity of 98% among pulmonary tuberculosis
- However, for varied applications, such as species identification and drug resistance detection, more sophisticated techniques such as culture and molecular tests need to be undertaken
Molecular Tests:
Xpert MTB RIF Assay
- Rapid and more sensitive detection of Mtb (Mycobacterium Tuberculosis) and RIF (rifampicin) resistance for MDR (multi-drug resistant) strains in both pulmonary as well as extra pulmonary TB with results available in 2 hours
- Over and above, appropriate treatment initiation, rapid results aid in accelerating the implementation of MDR-TB control measures, and ultimately reducing TB case incidence
- Xpert MTB/RIF pooled sensitivity was 98% in smear-positive and 67% in smear-negative pulmonary TB cases
- The sensitivity, specificity of Xpert MTB/RIF assay for diagnosis of Extra Pulmonary (EPTB) cases were 84.91% and 86.72%
- However, due to limitation of drug resistance detection of only Rifampicin, more sophisticated technique such as (LPA) Line Probe Assay has been introduced
Line Probe Assay
- Determines the drug resistance pattern of TB strains by the binding of DNA amplicons to the probe that targets resistance associated mutations to both first line as well as second line drugs
- LPA can be performed on DNA extracted from Culture or Direct Sputum
- First Line LPA (FL-LPA) assay (GenoType MTBDRplus V2,) showed a sensitivity and specificity for the detection of Rif resistance of 96.7% and 98.8%, respectively, and for the detection of H resistance, a sensitivity and specificity of 90.2% and 99.2%, respectively
- Second Line LPA (SL-LPA) assay (GenoType MTBDRsl V1 & 2) showed a pooled sensitivity and specificity for the detection of fluoroquinolone resistance by direct testing of 86.2% and 98.6%, respectively, and a pooled sensitivity and specificity for the detection of second-line injectables drugs resistance of 87.0% and 99.5%, respectively
- Limitations of the test as it depends on detection of specific genes for drugs and NOT ALL gene loci will be detected
Whole Genome Sequencing:
| Parameter | Whole Genome Sequencing of MT |
|---|---|
| Test application | Diagnosis + Drug Resistance |
| Strain Typing | Yes |
| Specimen requirement | Direct Sputum |
| Drugs for which resistance testing can be done | All (done in a single sequencing cycle) |
| Novel drug resistance mutations coverage | Yes |
| Turn-around time | 15 working days |
| Sensitivity | 100% (compared to Line Probe Assay as reference standard)[5] |
| Specificity | 98.04% (compared to Line Probe Assay as reference standard)[5] |
References
- Approaches to improve sputum smear microscopy for tuberculosis diagnosis, expert group meeting report geneva: 31 october 2009. (https://www.Who.Int/tb/laboratory/egmreport_microscopymethods_nov09.Pdf).
- Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults (Review). Horne DJ et.al., Cochrane Library, Cochrane Database of Systematic Reviews.
- Diagnostic Accuracy of Xpert MTB Compared to Smear Microscopy in Pulmonary vs Extrapulmonary Tuberculosis. Niveditha S1, Jagmohan S V2, Abhishek K Verma3, Minni Meka4. IJCMR., 2019.
- Line probe assays for drug resistant tuberculosis detection. Interpretation and reporting guide for laboratory staff and clinicians. (www.stoptb.org/wg/gli).
- Soundararajan L et al. Whole genome enrichment approach for rapid detection of Mycobacterium tuberculosis and drug resistance-associated mutations from direct sputum sequencing. Tuberculosis 121 (2020) 101915.
Brought to you by MedGenome-Dr Iravatham's Centre of Excellence
Testing Partners: Dr Iravatham's Clinical Laboratory 

Get Genetic Counseling with MedGenome Genetic Experts
Please share your details with our genetic experts to answer your queries


 Enquire
Now
Enquire
Now