What is Molecular Testing for Hematological Malignancies?

A set of a wide range of tests offered to understand the molecular and genetic basis of leukemias in order to make more informed treatment-related decisions. A combination of various techniques and multiple markers tested by each technique that provides a comprehensive understanding of a patient’s leukemia to the treating clinician.

Prevalence

Leukemias are 7th most occurring cancers in terms of incidence as well as mortality rate
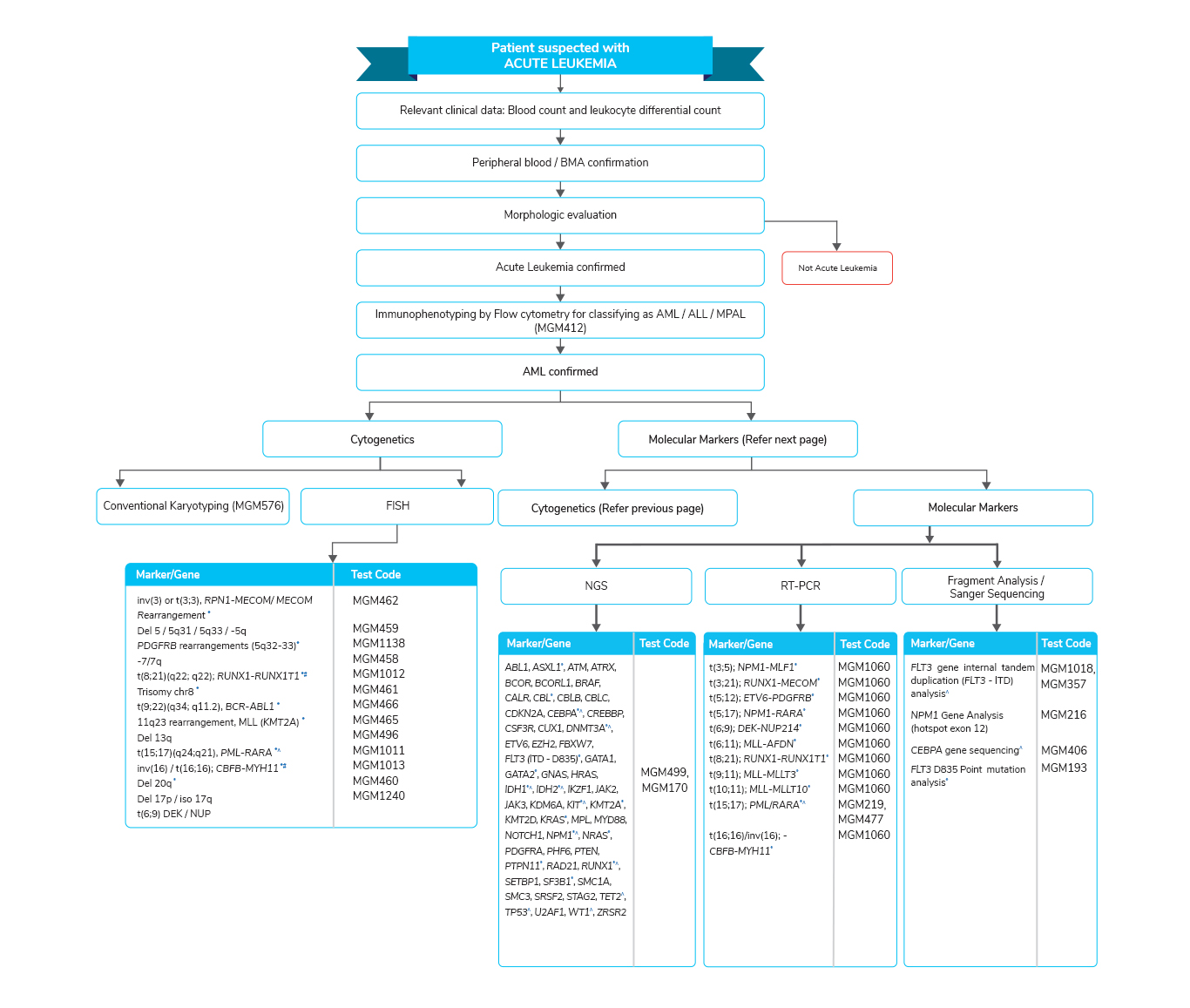
Acute Myeloid Leukemia (AML)- The most common type of leukemia in adults
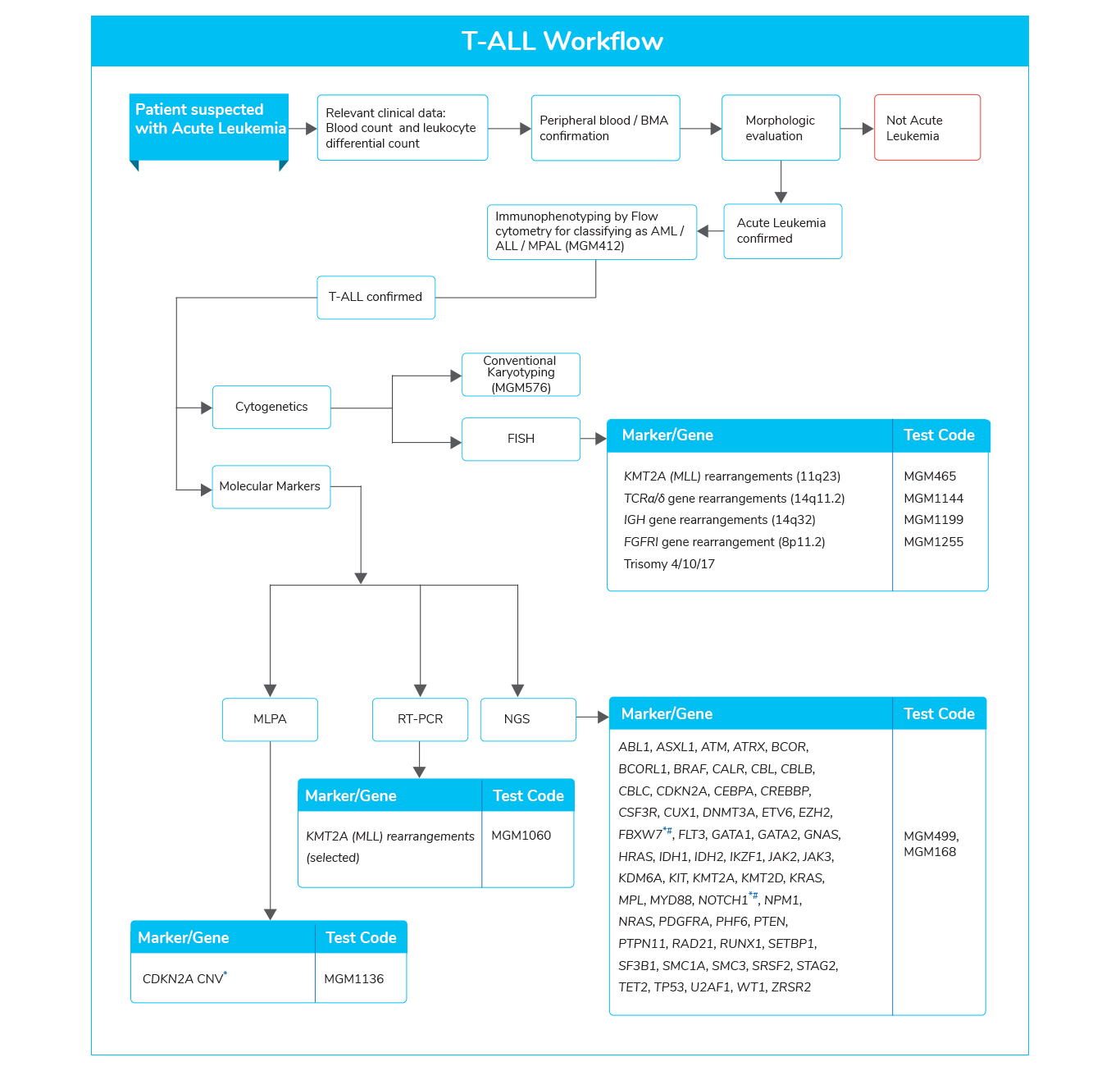
Acute Lymphoblastic Leukemia (ALL)
- The most common malignancy in children
- Accounts for 75% of all childhood leukemia and 25% of all childhood cancers
Chronic Myeloid Leukemia (CML)- Accounts for 15-20% of diagnosed leukemia cases in adults
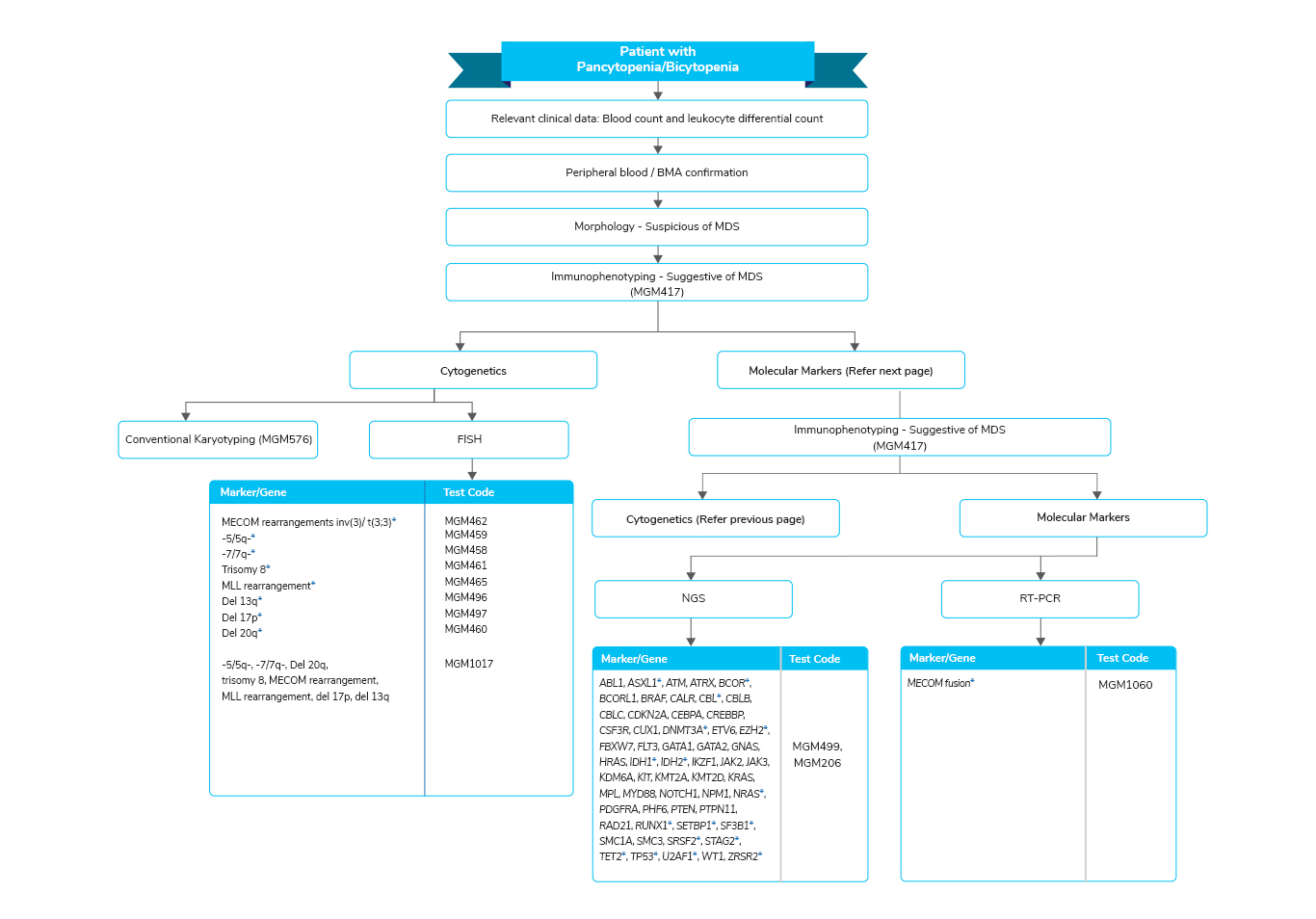
Myelodysplastic Syndrome (MDS)
- A disease of the elderly with a median age of 70 years
- In India, the incidence of MDS is unknown, however, the median age of diagnosis is less than 60 years, which is lower than what is seen in the Western population
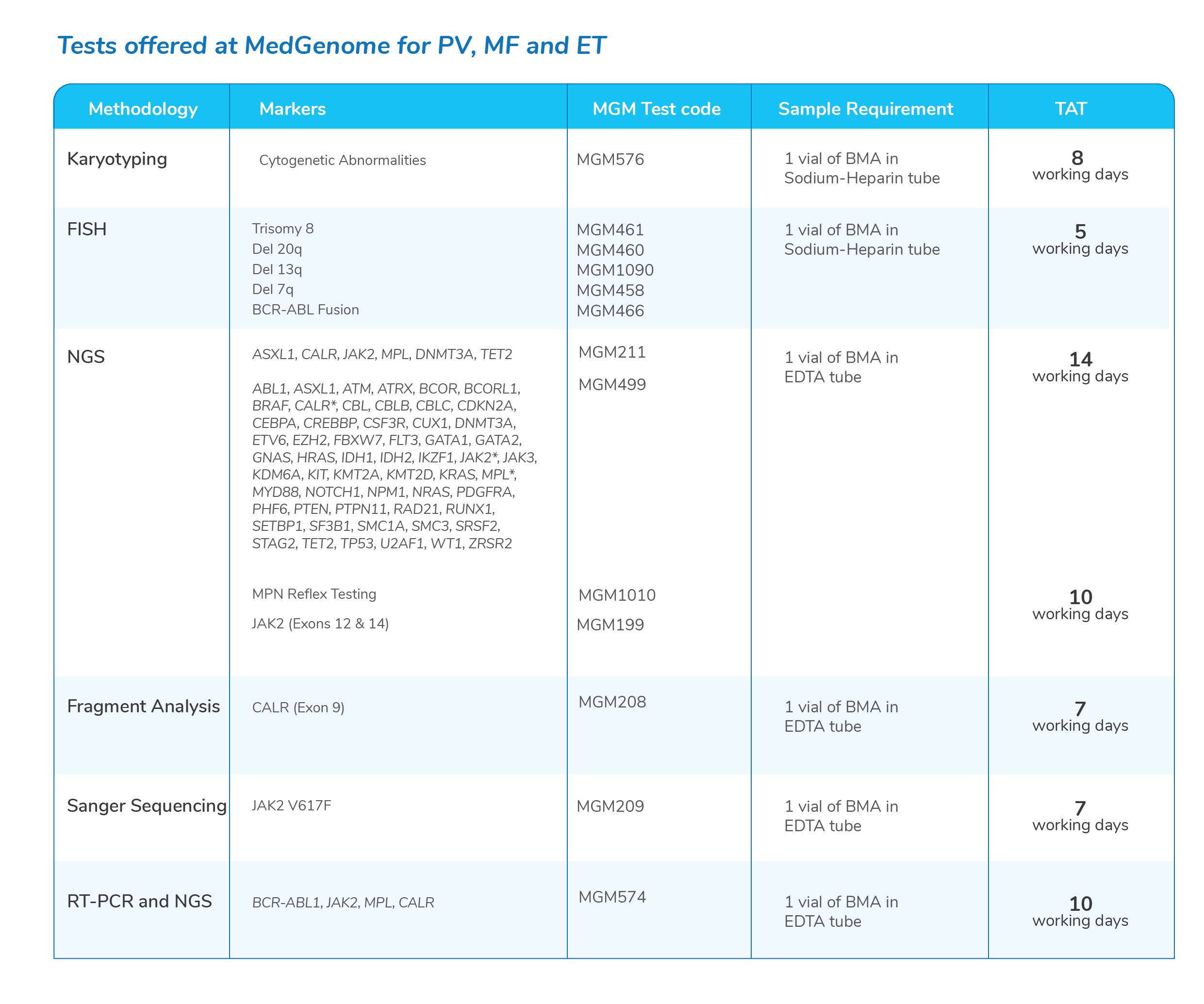
Myeloproliferative Neoplasms (MPN)- More commonly diagnosed in people over the age of 50 years and rarely occurs in young people
Most Common Leukemias
- Acute Myeloid Leukemia
- Chronic Myeloid Leukemia
- Acute Lymphoblastic Leukemia
- Chronic Lymphocytic Leukemia
- Myelodysplastic Syndromes
- Myeloproliferative Neoplasms
Why do you need the test?
The comprehensive multigene panel provides the advantage of understanding the clonal drivers at the time of presentation and during the course of disease evolution, thus helping in more informed treatment decisions in most of the leukemias, primarily for risk stratification and targeted therapy.
- The response to treatment and overall survival of patients with AML is heterogeneous
- Several prognostic factors related to patient and tumor characteristics have been described for AML
- Mutations in specific genes are found in many cases of AML and can be identified using genetic tests
- The results of the tests can be used for diagnosis and patient risk stratification
- Treatment of AML is based on risk stratification, mainly patient age and tumor cytogenetics
- In patients with cytogenetically normal AML, the identification of mutations in several genes, including FLT3, NPM1, and CEBPA, have been proposed to allow for further segregation in the management of this heterogeneous disease
- The most common mutations found in AML include FLT3, NPM1, DNMT3A, NRAS, CEBPA, TET2, WT1, IDH2, IDH1, and KIT.
- Molecular mutations have been analyzed to subdivide AML with normal cytogenetics into prognostic subsets.
- Confirmatory diagnosis and identification of important prognostic and predictive biomarkers to tailor therapy
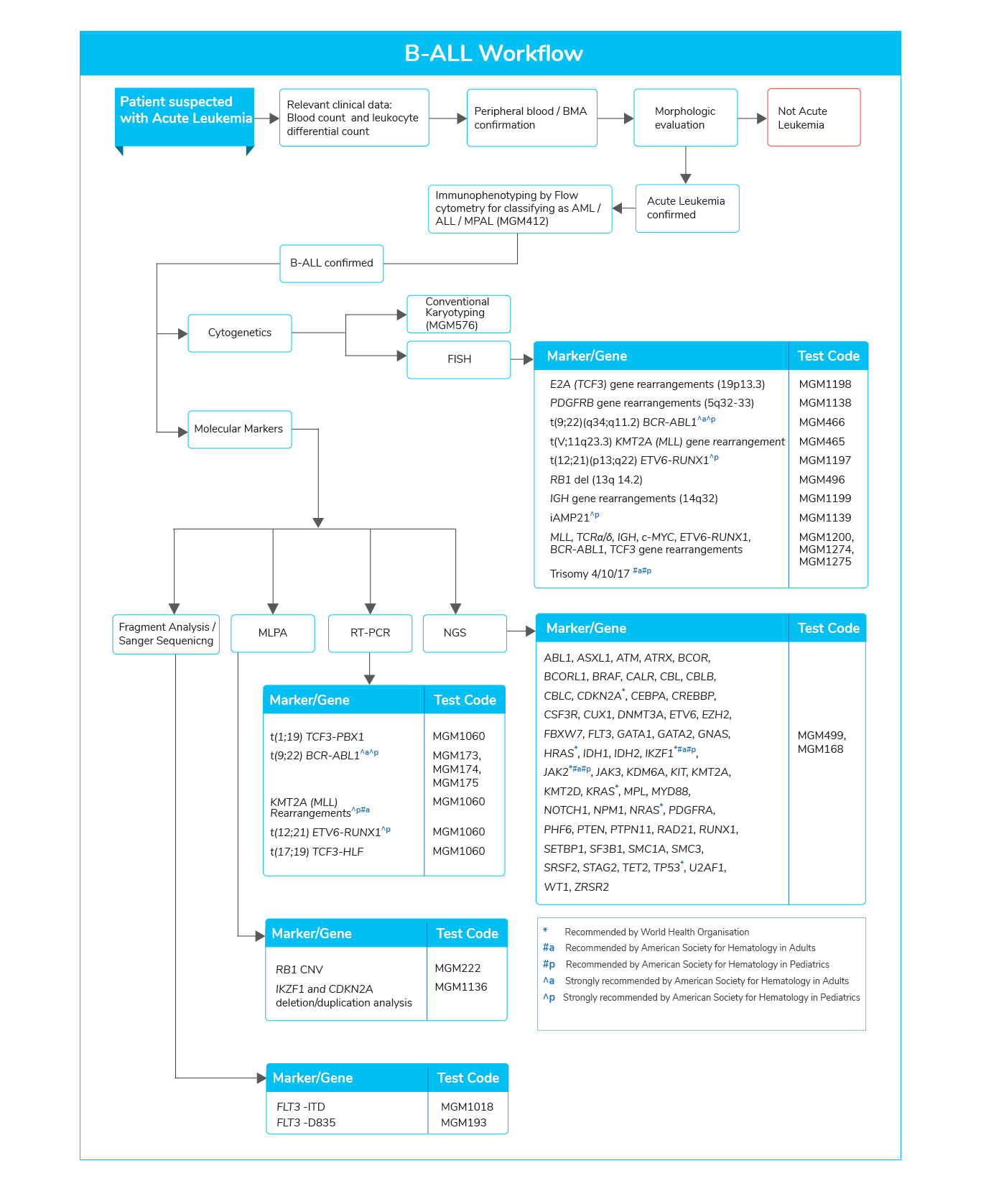
- Mutations involved in various key pathways are found in different subtypes of B-ALL. Genes related to various pathways such as:
- Transcriptional regulators (IKZF1)
- Cell-cycle regulation and tumor suppression (TP53 and CDKN2A)
- Tyrosine kinase receptor signaling (ABL1, FLT3, JAK2)
- RAS signaling (NRAS, KRAS, and HRAS)
- Epigenetic modification (CREBBP)
- Among them, specific genetic alterations are found to be associated with adverse clinical outcomes and increased risk for relapse
- Analysis of these genetic lesions enables the clinician to accurately assess the patient, considering age for precision medicine, predict the outcomes, and the possibility of allogeneic stem cell transplantation (Allo-SCT).
- The mutational analysis may further aid in the diagnosis, evaluating response to treatment using minimal residual disease (MRD) and risk assessment.
- Failure in treatment of CML is often associated with poor compliance due to BCR-ABL1 mutation (resulting in altered drug binding) and acquisition of other gene mutations eventually leading to TKIs resistance
- Mutations in genes such as IKZF1 and CDKN2A are classical mutations implicated in >50% of BP-CML patients.
- Therefore, analysis of the network of genes aids in improving the prognosis and therapeutic relevance by assessing for risk-associated therapy and prevention of progression
- During treatment BCR-ABL1 transcript levels may go undetectable, which may discourage the patient to follow the therapy
- Discontinuing TKIs was found being risky as rapid recurrence has been observed in 60% of CML patients. Therefore, molecular testing also enables detection of minimum residual disease (MRD) for clinical evaluation of therapy
- For decades, ‘watchful waiting’ has been the standard of care for patients with early-stage CLL
- This approach has focused on minimizing toxicity for the minority of patients whose disease may never evoke clinical symptoms
- Considering the clinical heterogeneity of CLL, genetic markers have been established as routinely used prognostic factors in addition to the traditional staging systems
- There is strong evidence that several of the predictive markers could influence treatment decisions for tailored therapy
- The predictive potential of genetic factors associated with CLL outcome is determined by
- Accessibility of the marker for routine use
- Stability of the marker throughout the course of the disease
- Sensitivity and specificity of the marker
- Some of the subtypes in MDS are associated with clinical biomarkers that aid in disease diagnosis and overall survival
- ETV6, DNMT3A, EZH2, CUX1, RUNX1, U2AF, and STAG2 mutations independently predict poor prognosis in MDS patients
- DNMT3A mutations were particularly found to be associated with RARS and lowest with RA subtype
- ]MDS patients with DNMT3A mutation displayed a higher risk of leukemia evolution and shorter overall survival
- TET2 is seen at a frequency of 20% in MDS and is a marker of a favorable prognosis
- SF3B1 mutation was found to be highest in RARS and lowest in RA subtype and has a favorable prognosis in MDS
- With a frequency of 5% to 15% in MDS, mutant RAS has a high likelihood of transformation to AML and shows an overall poor prognosis
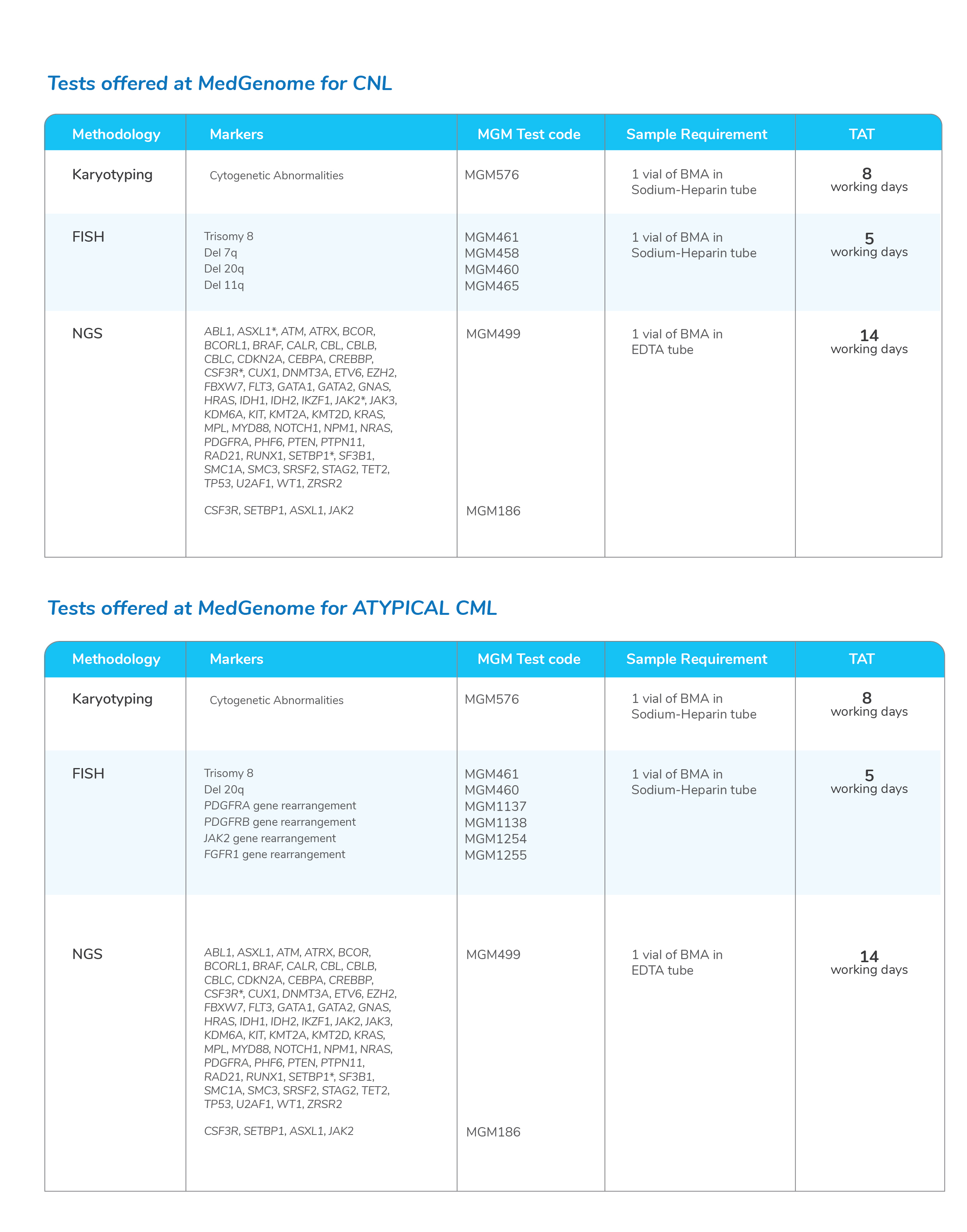
- With a unique pathogenesis and treatment, CML is often considered separately from the rest of the MPNs
- The most commonly recognized mutation in the remainder of the Philadelphia chromosome-negative MPNs is Janus kinase 2 (JAK2) V617F, which is present in more than 90% of patients with Polycythemia vera and approximately half of those with primary myelofibrosis or essential thrombocythemia
When do you need to get tested?
MedGenome tests cover markers that are of diagnostic, prognostic, and therapeutic importance. Based on individual leukemia subtype there are various markers to be evaluated according to a work-up by bodies like World Health Organization (WHO) and American Society for Hematology (ASH). The diagnostic markers can be tested during the presentation of the disease, whereas prognostic and therapeutic markers can be tested while making therapeutic decisions.
Who needs to get tested?

- Patients with leukemia or suspected to have leukemia, to get insights on differential diagnosis, prognosis, targeted treatment, drug effectiveness, relapse monitoring as well as for minimal residual disease estimation (for investigational use only).
Why MedGenome?
MedGenome offers a range of tests for different types of leukemia. Covers a wide range of diagnostic, prognostic and therapeutic markers for the clinician to have deep insights into their patients’ leukemia. Various testing techniques include
- Next-generation Sequencing (NGS)
- Sanger Sequencing
- Real-time PCR (RT-PCR)
- Fluorescent In-Situ Hybridization (FISH)
- Multiplex Ligation-dependent Probe Amplification (MLPA)
- Fragment Analysis
- Flow Cytometry
- Karyotyping
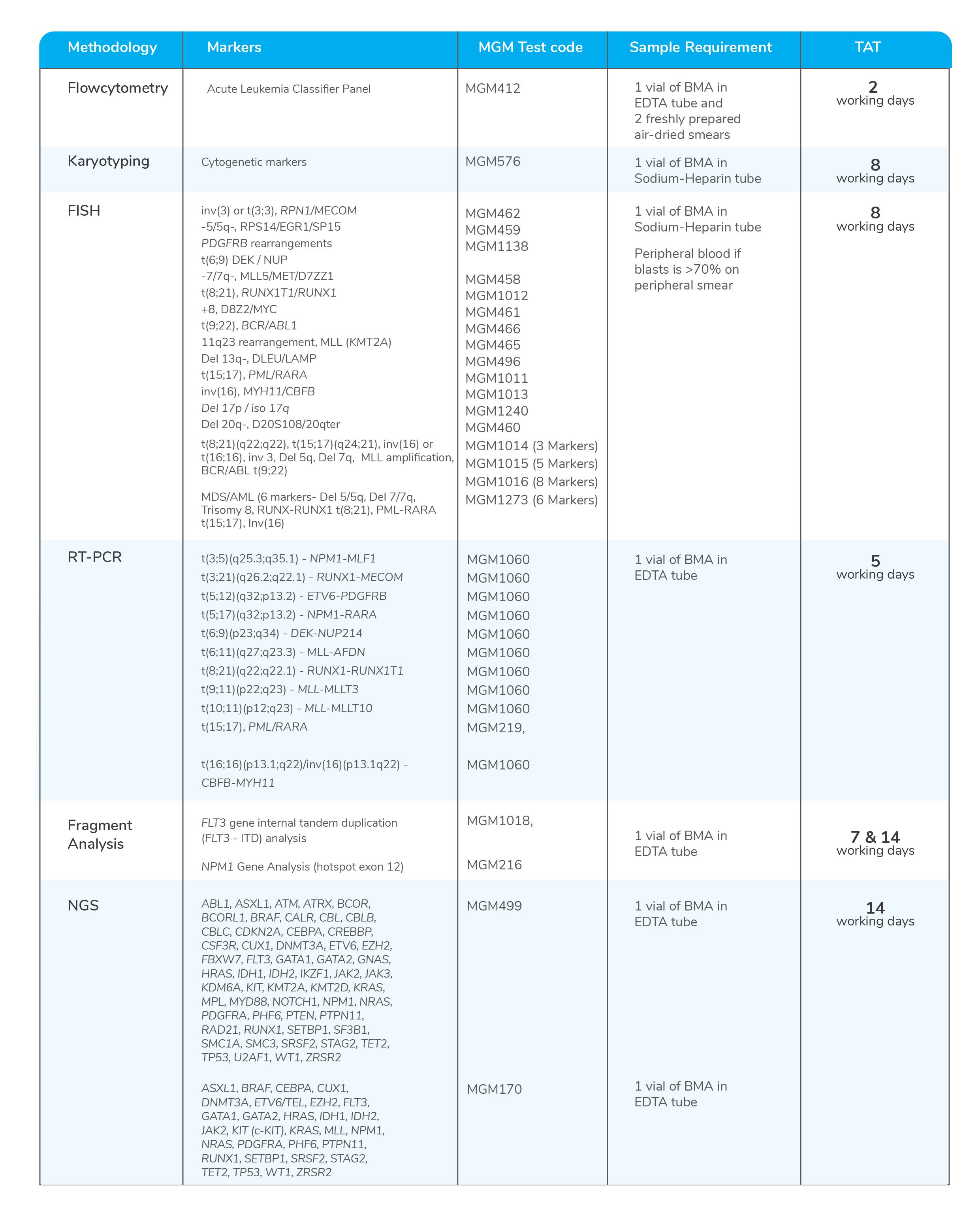
Molecular tests for Acute Myeloid Leukemia
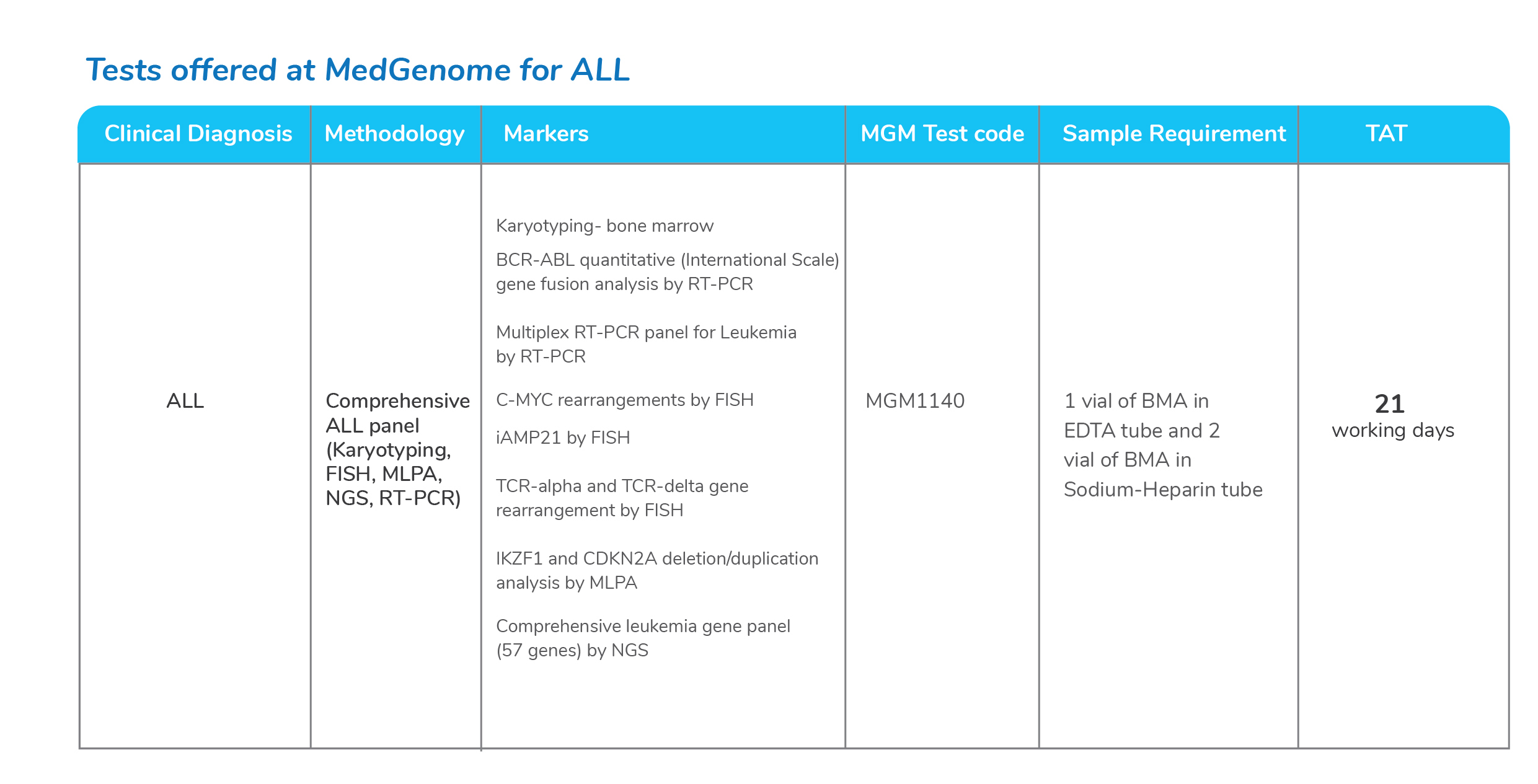
Molecular tests for Acute Lymphoblastic Leukemia
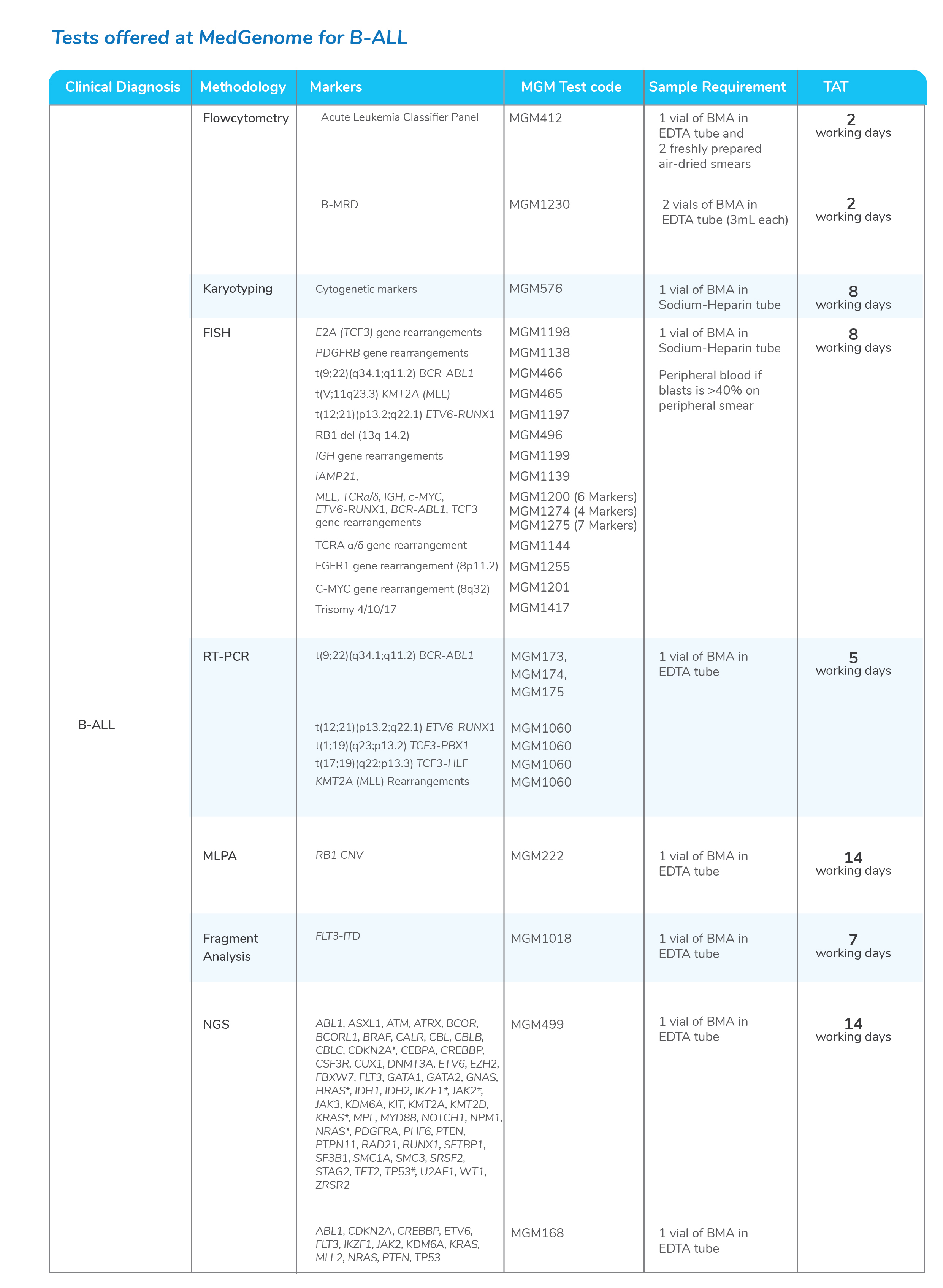
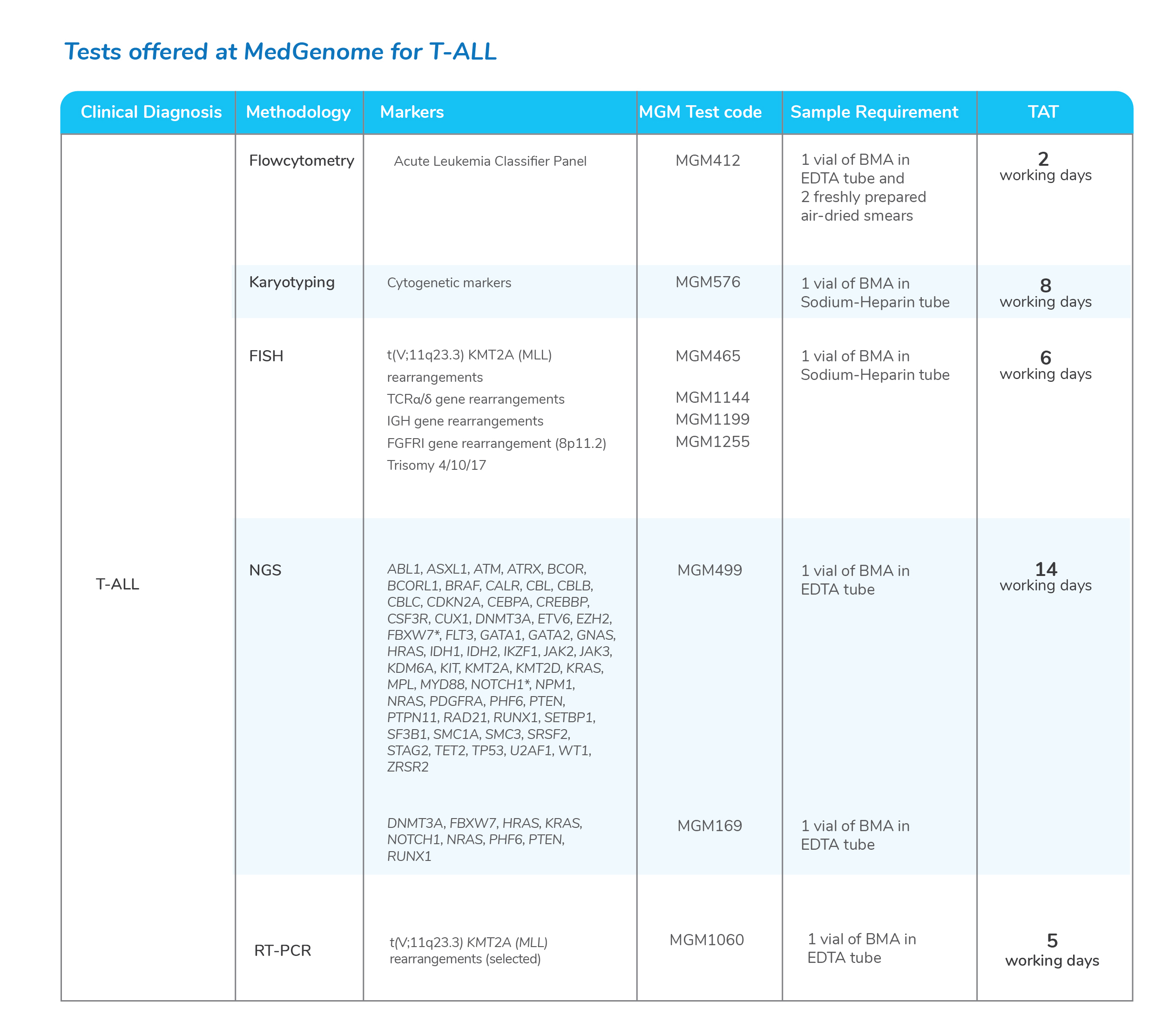
Molecular tests for Chronic Myeloid Leukemia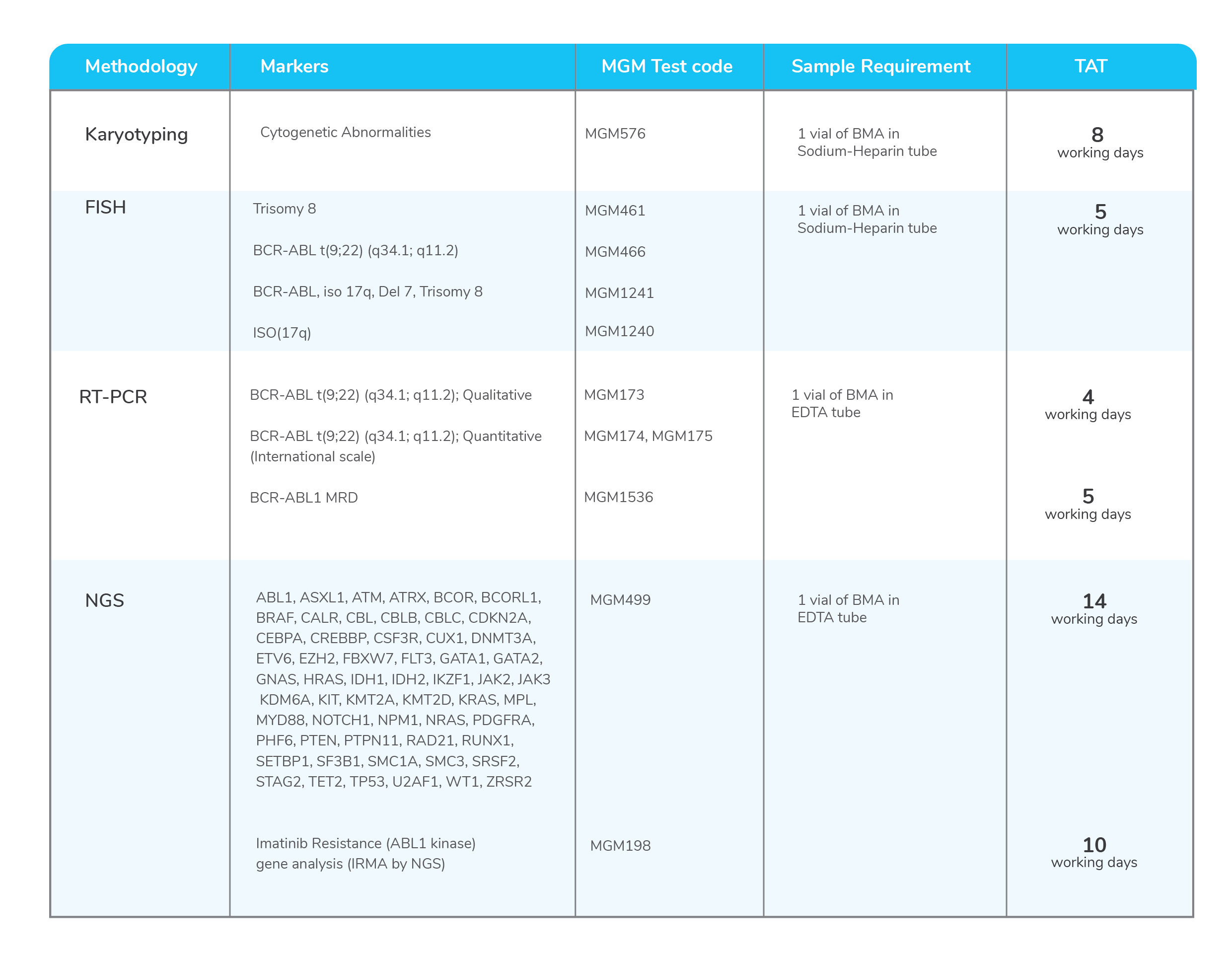
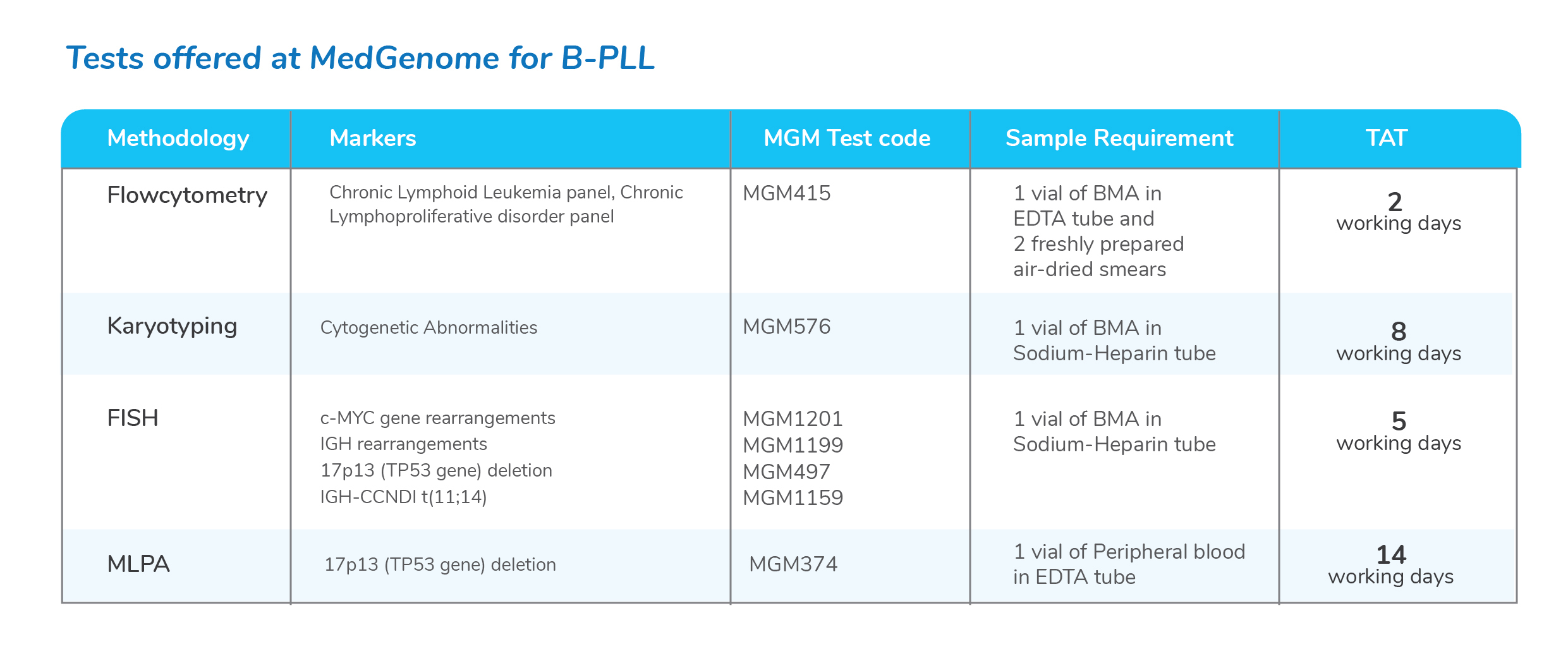
Molecular tests for Chronic Lymphoid Leukemia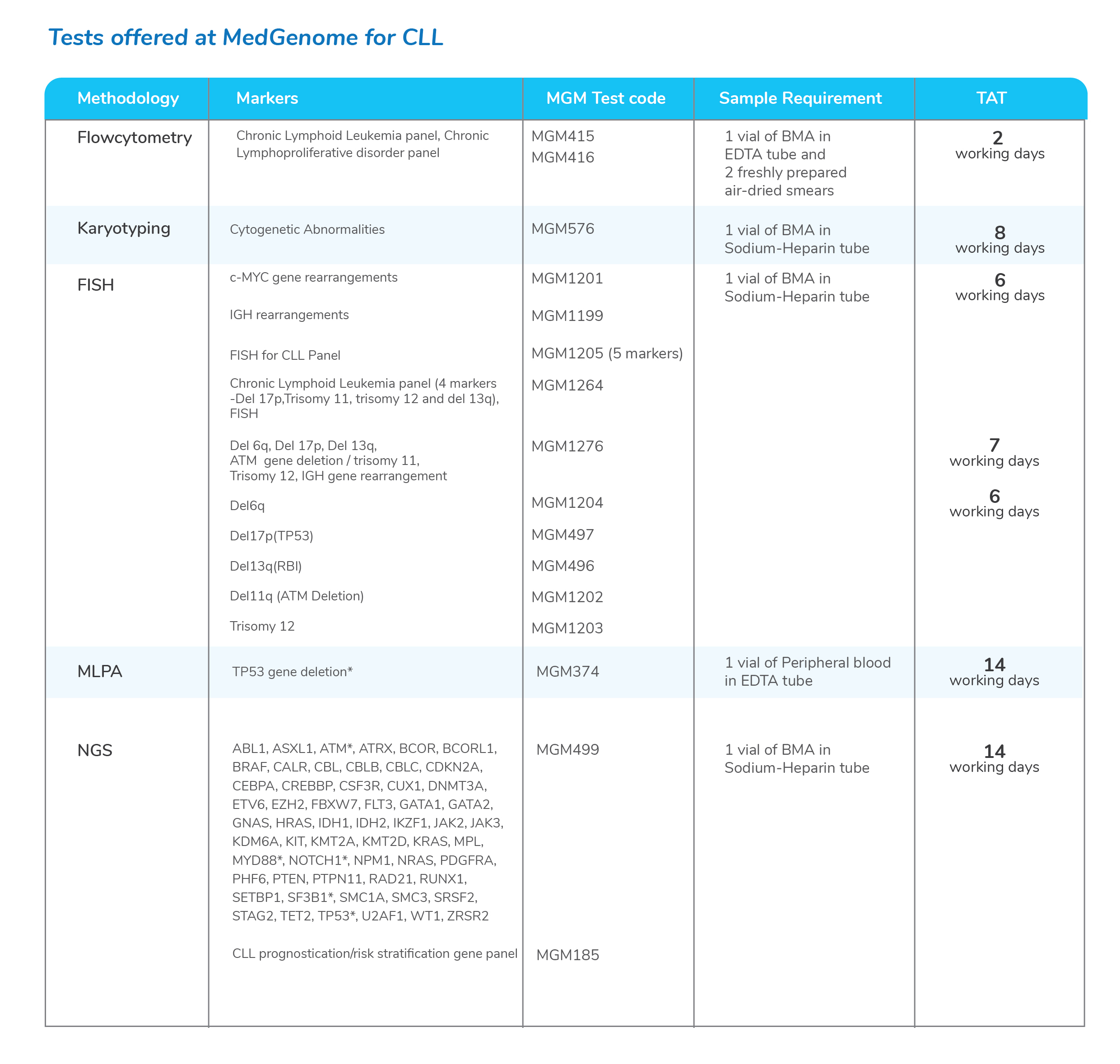
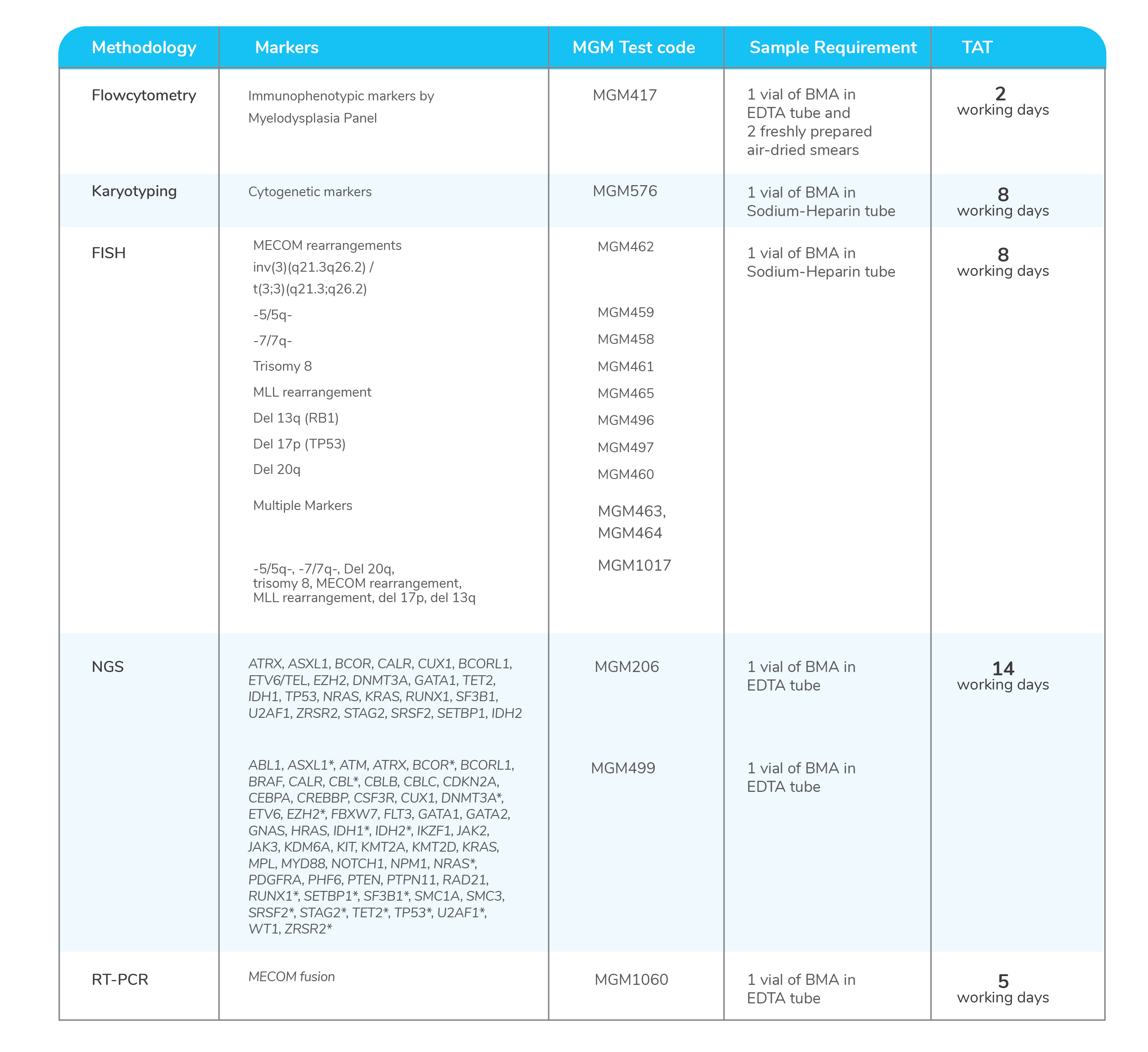
Molecular tests for Myelodysplastic Syndrome
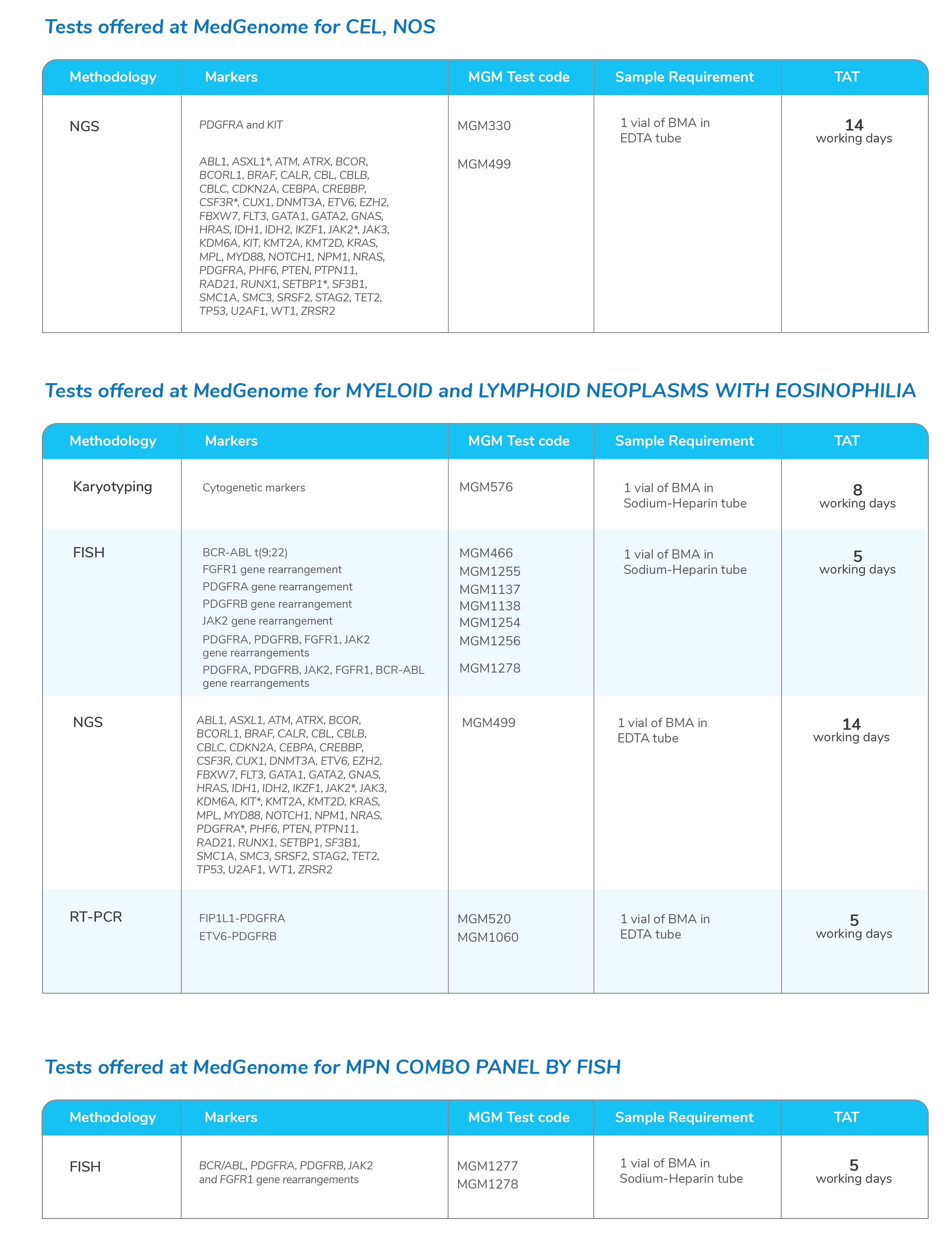
Molecular tests for Myeloproliferative Neoplasms
Test offered at Comprehensive Leukemia panel
| Test Code | Test Name | Method | TAT (Working days) | Test Location |
|---|---|---|---|---|
| MGM2450 | Comprehensive Leukemia panel (SNVs, small
INDELs, CNVs and Fusions) Inclusive genes/Special Instructions: SNVs, Indels and CNVs in 133 genes (ABL1, ASXL1, ATM, ATRX, BCOR, BRAF, CALR, CBL, CBLB, CBLC, CDKN2A, CEBPA, CREBBP, CSF3R, CUX1, DNMT3A, ETV6, EZH2, FLT3, GATA1, GATA2, GNAS, HRAS, IDH1, IDH2, IKZF1, JAK2, JAK3, KDM6A, KIT, KMT2A, KMT2D, KRAS, MPL, MYD88, NOTCH1, NPM1, NRAS, PDGFRA, PHF6, PTEN, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC1A, SMC3, STAG2, TET2, TP53, WT1, ZRSR2) 600+ Fusions are tested (Fusions/Gene list will be provided on request) Bone Marrow morphology report, Complete blood count report and/or FISH report if available should accompany the sample | NGS | 12 | Bangalore |


 Enquire
Now
Enquire
Now