
Comprehensive CAP-accredited Liquid Biopsy Portfolio
Complementary, Alternative, Reflex and Serial to the Tissue Biopsy test
What is Liquid Biopsy Test?
Dying cancer cells release their mutated DNA into the bloodstream, enabling determination of mutation type and relative tumor volume. In this non-invasive technique, by capturing and sequencing the tumor-derived cell-free DNA, tumor genomic profile can be reconstructed without needing to perform a biopsy of the tumor. This process is also called liquid biopsy which is designed to sequence regions of oncogenes.
MedGenome Liquid Biopsy NGS Assays

Why do you need the test?

- Facilitates early detection of emergent genetic alterations that can be associated with resistance to therapy during cancer progression
- The quantitative assessment for the disease is based on Mutant Allele Frequency
The advantages of this test using Next-generation Sequencing (NGS) technology are as follows:
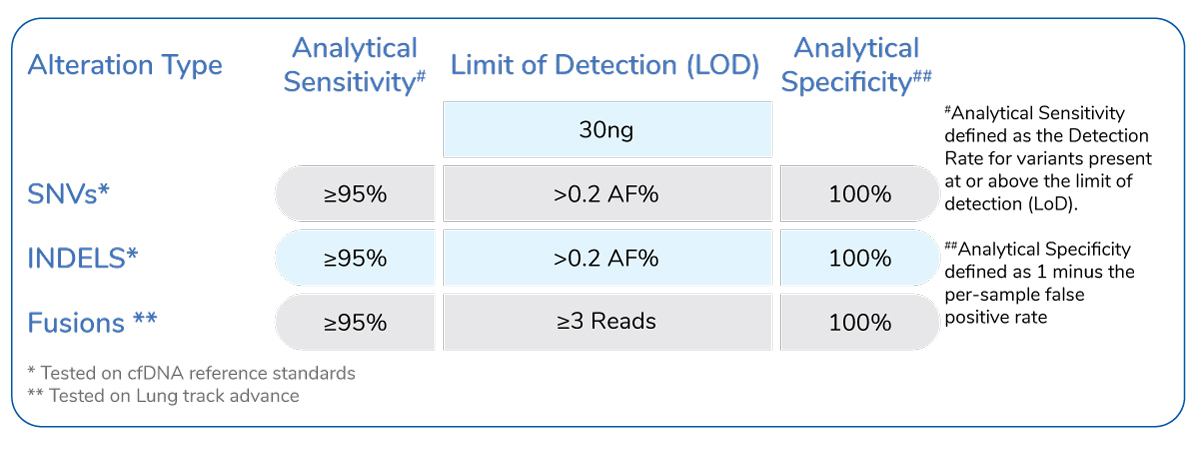
- High sensitivity and ability to detect low-frequency mutation
- Unlike RT-PCR, NGS has the advantage of multi-gene profiling in a single assay providing on the exon covering HOTSPOT mutation, with a minimal amount of DNA without compromising on the sensitivity and specificity
- Reduced turn-around time
Liquid biopsy facilitates the detection of clinically relevant tumor mutations from a blood sample when it is difficult to obtain a biopsy for practical and clinical reasons:
- Sample quantity and frequency is not limited, contrary to most biopsies
- Simple and safe venipuncture that doesn’t require a complex procedure
- Doesn’t require urgent shipping/cold-chain support
When do you need to get tested?

- Treatment and relapse monitoring in cancer patients
- Understanding secondary acquired resistance mutations during the course of treatment
- Monitor patients during the initiation of baseline therapy and at regular intervals for assessment of clinical response to the treatment
Who needs to get tested?
Cancer patients who fall in one of the following categories can get tested with liquid biopsy
- The biopsy material is degraded or damaged or poorly fixed
- The tumor content is insufficient in the existing biopsy material
- No available tumor tissue biopsy
- For those patients whose clinical condition is not suitable for an invasive procedure
Disclaimer: Despite the advantages, liquid biopsy is an investigational test. It is not meant for diagnostic purposes and does not replace conventional biopsy.
Why MedGenome?
- MedGenome liquid biopsy test (ONCOTRACK and ONCOTRACK ULTIMA) detects the presence of oncogenic driver mutations.
- This has a potential role in clinical decision making for different lines of approved targeted therapy and those in clinical trials.
- This assay also screens for resistance mutations that demonstrate the mechanism of acquired secondary/primary resistance to these drugs
- Developed on an NGS platform and is highly sensitive and can accurately detect low-frequency mutations


 Enquire
Now
Enquire
Now