What is Non-invasive RhD testing?

Non-invasive prenatal test (NIPT) of fetal RhD status uses a real-time quantitative polymerase chain reaction (PCR) method to detect fetal RhD status using cell-free fetal DNA in RhD negative women. MedGenome brings this innovative non-invasive solution which enables that prophylaxis is administered only to those rhesus D-negative women who carry a RhD-positive fetus.

Why RhD testing is required?
- RhD negative pregnant women may carry a fetus with RhD positive blood group which may cause sensitisation, any time during the pregnancy, but it is most common in the third trimester and during childbirth.
- This itself has no adverse effects on the mother and does not usually affect the pregnancy during which it occurs.
- However, in a subsequent pregnancy with a RhD-positive fetus in women who have been sensitized to the RhD antigen, the woman’s anti-D antibodies may cross the placenta resulting in haemolytic disease of the fetus and new-born.
- Knowing the fetal RhD status helps decide management of each pregnancy, especially the need to administer prophylaxis with anti-RhD immunoglobulin to reduce the risk of the sensitisation in RhD-negative women and hence the prevalence of haemolytic disease of the fetus and new-born in subsequent pregnancies.
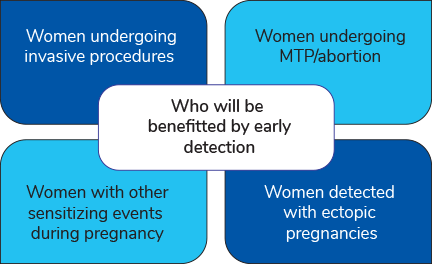
Who can benefit from NIPT Rhesus D Track?

- Pregnant women with confirmed Rh-D negative status.
- Above gestational age of 11 weeks.
- Carrying a singleton pregnancy.
NIPT Rhesus D Track will benefit in early detection of Fetal RhD status to support Clinicians for better and effective management.
Test Sample Requirement for RhD
10 ml of maternal peripheral blood via an StreckTM tube.
Turn-around Time (TAT):
14 days
Get Genetic Counseling with MedGenome Genetic Experts
Please share your details with our genetic experts to answer your queries


 Enquire
Now
Enquire
Now